Introduction: Orelabrutinib is a second-generation Bruton tyrosine kinase inhibitor (BTKi) with high kinase selectivity. Clinical studies have demonstrated that orelabrutinib achieved a high complete response rate of 26.3% in relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), showing promising efficacy and acceptable safety profile. However, the real-world data of Orelabrutinib are not available. This study aimed to evaluate the effectiveness and safety of orelabrutinib-containing regimens for the treatment of patients (pts) with CLL/SLL in a real-world setting.
Methods: This was a retrospective, multi-center, real-world study conducted. Pts (aged 18 years or older) with CLL/SLL who received at least 3 months of orelabrutinib-containing regimens between December 2020 and March 2023 were analyzed. Efficacy was evaluated by hematologic response rate (HRR), defined as the proportion of pts who met at least 1 of the following hematologic response criteria: hemoglobin response (pts having a recovery of hemoglobin value or ≥50% increase from baseline), platelet response (pts having a recovery of platelet count or ≥50% increase from baseline), or lymphocyte response (pts having a recovery of lymphocyte count or ≥50% reduction from baseline). Safety was assessed by adverse events (AEs) as per NCI-CTCAE 5.0.
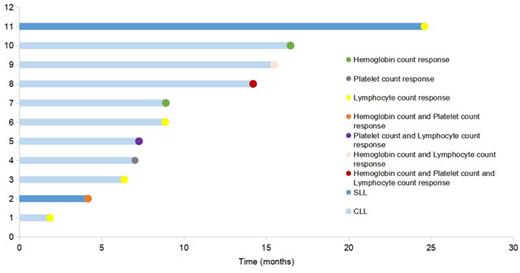
Results: 24 pts with CLL/SLL (12 males; 22 CLL) were included, with a median age of 62.5 years (range: 38-82). Most pts had an Eastern Cooperative Oncology Group score of 0-1 (17/24, 70.8%) and Rai stage III or IV disease (16/18, 88.9%). 7 pts with hypertension, of which 2 with very high-risk hypertension. 3 pts have previously received other BTKi. A total of 9 pts(37.5%) performed del(17p), TP53, or IGHV testing. Among them, pts received orelabrutinib 100 mg (2/24,) or 150 mg (22/24,) once daily. As of the cut-off date (July 13, 2023), the median follow-up was 10.67 (range, 2.73-25.77) months. 18 pts (16 CLL, 2 SLL) with abnormal hemoglobin value, platelet count, or lymphocyte count at baseline were evaluable for hematologic response. Hematologic response was achieved in 11 (61.1%) of 18 evaluable pts. Pts aged <65 years had a higher HRR versus pts aged ≥65 years (87.5% vs. 40.0%, p=0.040). HRR was 56.3% in CLL versus 100.0% in SLL pts, and 60.0% in pts with hypertension versus 61.5% without hypertension . Four of 11 responders had a hematologic response in at least two criteria (CLL, n=3; SLL, n=1), of which 3 pts had a hemoglobin response (Figure 1). The most commonly reported AEs included hemoglobin decreased (16.7%) and platelet count decreased (16.7%). Grade ≥3 AEs were white blood cell count decreased (4.2%), platelet count decreased (4.2%), and lymphocyte count decreased (4.2%). No pts experienced severe AEs. No cardiovascular AEs were reported during treatment in 7 pts with hypertension at baseline.
Conclusions: This real-world study assessed the preliminary characteristics and clinical outcome of patients receiving orelabrutinib-containing regimens under routine clinical practice conditions in China. The orelabrutinib-containing regimens in this study furnished beneficial evidence for the treatment of CLL/SLL and is essential for determining the best treatment option, future data with a larger sample and longer follow-up will be updated.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal